EPR in Condensed GasesOverview of the experimental technique and results.Since the late eighties the research activity of the laboratory in the field of matrix isolation has been focused on the EPR study of trapped light atoms and radicals. A contribution has been made to the data of matrix shifts of the EPR spectra parameters for hydrogen, H, deuterium, D, nitrogen, N, atoms and methyl, CH3, and formyl, HCO, radicals and their isotopomers isolated in rare-gas and molecular solids. The deposition from the gas phase on to a cold surface of a quartz finger inserted into the EPR microwave cavity has been utilized to obtain the samples. Two gas flows were provided to the substrate: one of the two was passed through a gas discharge tube where the radicals were formed and another one was set avoiding the gas discharge zone. Shown below is one of the schemes of the major section of the experimental set-up: the microwave cavity of the 3 cm EPR spectrometer with 100 kHz modulation of the magnetic field, the low temperature gas discharge device and the quartz finger.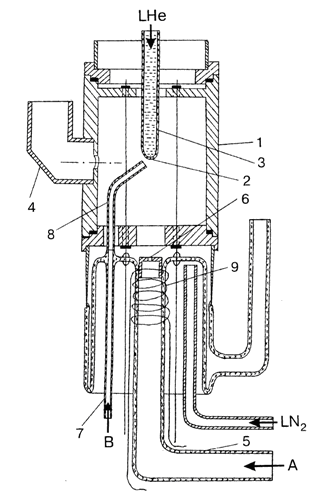
FIG.1.
Here 1 is the cylindrical TE011-mode cavity, 2 is the bottom of the quartz finger 3, filled with liquid He, and 4 is a waveguide. In order to obtain temperatures above 4.2 K,
the bottom can be cooled bt liquid He vapor. An electrodeless RF gas discharge
is excited in the glass tube 5 with outlet 6 of 0.6 mm diameter. The matrix gas can be supplied to substrate 2 by glass tube 7 and further by quartz tube 8 inserted
into the cavity (channel B). The end of the tube 8 is located close (3 mm) to the bottom 2 which facilitates effective freezing out of the matrix gas.
The whole device presented in the figure is cooled externally with liquid nitrogen vapor (LN2) and its temperature can be varied from 77 to 300 K.
A high-frequency (15 MHz) oscillator is used
to maintain the discharge. The high-frequency power is fed through a coaxial cable to coil 9 wound over discharge tube 5.
The experimental technique described above made possible to obtain, for the first time, H and D atoms matrix-isolated from the gas phase in solid Ne [1,2]. Thorough experimental study of H and D atoms trapped in various matrices and analysis of both the data obtained in the study and those available from the literature led to the observation and explanation of the isotope effect in the hyperfine constant matrix shifts and EPR linewidths of the atoms in H2, D2, N2, Ne, Ar, Kr and Xe matrices [3-6]. Not only trapped particles but matrices themselves were investigated. Nitrogen atoms isolated in n-H2 matrix were utilized for the first time to observe and study by EPR technique ortho-para conversion in the normal solid hydrogen stimulated by a paramagnetic impurity [7,8]. In the case of N atoms in N2 matrix, careful analysis of the EPR experimental data obtained showed that two different types of equilibrium surroundings are possible for a nitrogen atom trapped in a substitutional position, one of which corresponding to an undistorted N2 crystal lattice, and the other to a position in which the axes of nearest neighboring molecules are directed towards the trapped atom, and the centers of gravity of molecules are displaced to the center of the matrix cell. A value of the displacement was assessed. In order to elucidate factors and processes which limit the concentration of trapped active particles, matrix isolation of nitrogen atoms in solid N2 was studied under wide range of the flow rates of nitrogen atoms and molecules fed onto the substrate. It was found out [9] that the major factor governing the concentration of impurity atoms was the atom surface diffusion during sample deposition which resulted in the recombination of these active particles. At present, the laboratory research follows three scientific directions in the field of matrix isolation.
Contacts: Dmitriev Yu.A.
PUBLICATIONS
|